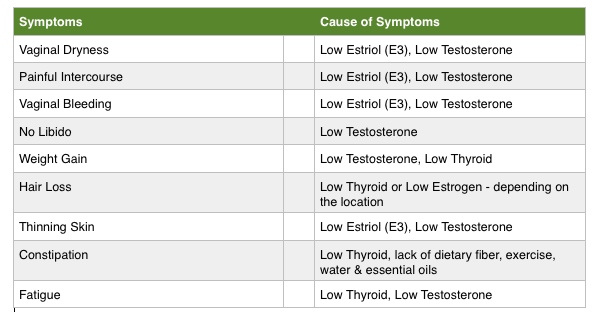
When Becky first came to see me she was 59. She looked younger than her age. However, Menopause made her feel much older than she looked. Over the last few years having intimacy with her husband had become the last thing on her mind because vaginal dryness and atrophy of her vaginal tissue made having sex excruciatingly painful. Her vaginal tissues had become so thin that it caused vaginal bleeding after intercourse and frequent urinary tract infections.
Here were Becky’s Chief Complaints at her Initial Office Visit:
Before Becky came to see me at the Hansen Clinic of Natural Medicine, she had tried taking Vagifem® (estradiol vaginal tablets), the conventional drug therapy for Vaginal Dryness and Painful Intercourse. The recommended dose for Vagifem® is once daily for 2 weeks followed by 1 tablet twice weekly. Becky’s symptoms were so severe that she used Vagifem® tablets as much as 5 times per day, but even that dose did not relieve her pain. Intercourse had become so severe that she could no longer endure the thought of having sex.
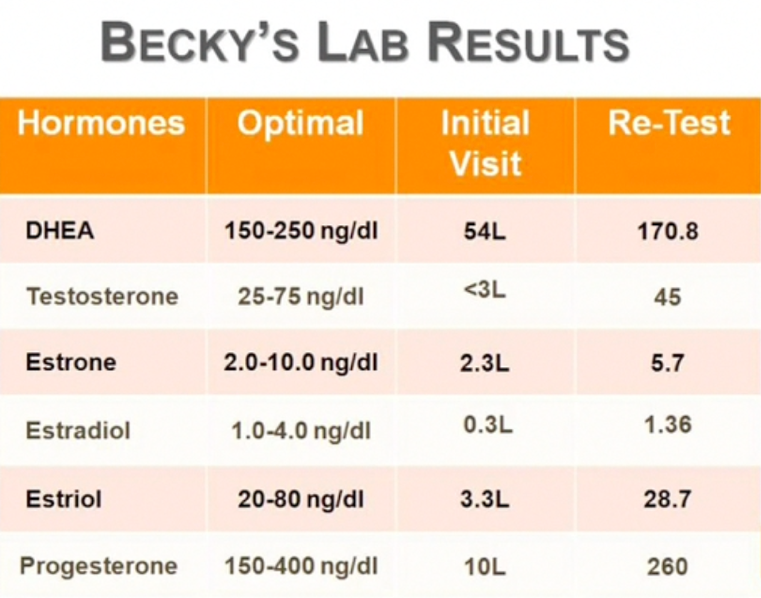
As you can see in the chart above, Becky was initially low in all 6 of the key hormones tested. However, after a few short weeks of supplementation with Bio-Identical Hormones, Becky’s levels had all been raised into the Optimal Levels.
At Becky’s 1 month follow-up, she reported that her vaginal dryness was better but still had some pain during intercourse. She said the pain in her vagina was inside at the roof of the vagina; that it was more pronounced deeper, when her spouse was fully inside and that it was still dry. However, she noted that her sex drive was up slightly, her energy was up, she had totally stopped coffee, she had lost weight and she was feeling much better overall.
One month later, Becky reported that for the first time in years she had absolutely no pain during intercourse: no pain and no sense of pressure either. She also said that her sex drive was markedly increased as well as her sexual sensitivity and lubrication.
The next time that I met with her, at her 4 month Follow-up appointment, Becky said that her sex drive was fabulous and that she no longer needed any lubrication before having sex because the Testosterone level was so good that her vaginal tissue was being stimulated to make so much natural lubrication that she didn’t need any additional. Needless to say, she was ecstatic with the results that she was feeling and so was her husband.
Make Sure You Read the Black Box WARNING for Synthetic Estrogens like Vagifem® and OSPHENA™
WARNING: ENDOMETRIAL CANCER, CARDIOVASCULAR DISORDERS, BREAST CANCER AND PROBABLE DEMENTIA

Fortunately, Becky gave up on Vagifem because it didn’t work and a friend who had been to see me told her she needed to learn about Bio-Identical Hormones, which were natural and didn’t come with the serious side-effects caused by the synthetic drugs, like Vagifem® and OSPHENA™.
OSPHENA™ is the latest drug approved by the FDA for the “Treatment of Atrophic Vaginitis due to Menopause.” It too, is an Estrogen look-a-like drug, which means it is a synthetic copy of Estrogen that so closely resembles Estsradiol that it binds to the same Receptors and causes some of the same actions, and side-effects.
On the “black box” WARNING that will feature prominently on the prescribing information insert for OSPHENA™, the lists of risks include the following: uterine cancer, strokes or blood clots, unusual vaginal bleeding, heart attacks, severe liver problems and breast cancer.
Other bothersome side effects that showed up in clinical trials included twice the rate of urinary tract infections, three times the rate of hot flashes, and 14 times the rate of yeast infections as the women on the placebo.
Who Paid for the Osphena Study?
Critics also note that the two 12-week trials that satisfied the FDA were funded by the drug’s developers, Shionogi, Inc. and QuatRx, a practice that is common but might give the public pause. Two of the lead authors, David J. Portman and James A. Simon, list so many consultancies, speakers’ bureau affiliations, and board memberships with pharmaceuticals (including Shionogi and QuatRx) that the disclosures take up half of the study’s first published page in Menopause, the journal of the North American Menopause Society. Also, The New York Times reported that Gloria A. Bachmann, another lead author, between 1998 and 2005 signed her name to ghostwritten papers that downplayed the risks of estrogen without disclosing that Wyeth had paid for them.
OSPHENA™ claims in its advertising that it is “the only FDA approved, NON-ESTROGEN, oral pill that actually improves certain vaginal tissue and significantly relieves moderate to severely painful intercourse due to menopause.”
That statement is a half truth. OSPHENA™ isn’t an estrogen; but it sure acts like one, and like estrogen-alone therapy, OSPHENA™ increases the risk of endometrial cancer. Stroke and deep vein thrombosis are also risks of taking the drug, albeit low ones compared to estrogen-alone therapy, says the FDA. These risks are detailed in a prominent black box warning on the drug.
Shortcomings of OSPHENA™ Clinical Trials
Some experts have questions about the clinical trials that led to the approval of OSPHENA™
- Two 12-week trials were funded by the drug’s developers; the lead authors have strong ties to the companies. Not unusual for drug trials but not exactly an unbiased effort.
- The degree of improvement after 12 weeks was less than half a percent.
- Benefit didn’t far outweigh harm, concluded another reviewer. Roughly, only 14% of the study’s subjects improved (over placebo), while another14% had significant adverse effects.
Alan Cassels, who researches drug policy at the University of Victoria and co-authored the book Selling Sickness with Ray Moynihan, looked over Osphena’s application and studies. “I’m amazed it got approved,” he said. While investigators report a statistically significant improvement for women’s “most bothersome symptom,” at 12 weeks that difference was less than half a percent between the women taking the drug and those on placebo. “You can make it sound very statistically significant, as they’ve done,” he said. “But how does that translate into something meaningful for a person’s life?” A company spokesman disagree but did not elaborate.
Barbara Mintzes, who studies drug marketing at the University of British Columbia, also gave the FDA application for Osphana a read. She notes that while roughly 14% of women (over placebo) had an alleviation of symptoms, a similar percentage of women also experienced an adverse event, like an infection. “This is not a positive ratio of benefit versus harm,” she wrote in a detailed email to Newsweek. Ideally, she says, you’d want to see the benefit outweigh the harm “by a good margin.” “These are very disappointing results—no one wants to take a medicine that is equally likely to make them worse as to make them better.” Shionogi disputes this analysis.
Critics also note that the two 12-week trials that satisfied the FDA were funded by the drug’s developers, Shionogi, Inc. and QuatRx, a practice that is common but might give the public pause. Two of the lead authors, David J. Portman and James A. Simon, list so many consultancies, speakers’ bureau affiliations, and board memberships with pharmaceuticals (including Shionogi and QuatRx) that the disclosures take up half of the study’s first published page in Menopause, the journal of the North American Menopause Society. Also, The New York Times reported that Gloria A. Bachmann, another lead author, between 1998 and 2005 signed her name to ghostwritten papers that downplayed the risks of estrogen without disclosing that Wyeth had paid for them.
What Does Dr. Hansen Say?
“I believe that his drug should never have been approved and would certainly not have been approved, were it not for the financial connections of the principal board members of the manufacturer and the principal researchers. This drug carries significant serious risks of cancer, stroke and heart attacks, while only 14% of women said that it improved their symptoms, and perhaps most importantly, it was only 0.5% better than the placebo. You don’t need to take risks with your health. Nature has provided a safer and more effective, natural alternative.
There is a Safer, More Effective Solution
Estriol is the “Safe Estrogen.” It is known to be 1000 times less potent than Estradiol in stimulating cellular proliferation that can lead to cancer. Receptor binding studies have also shown that Estriol has only low relative binding affinity to uterus endometrial estrogen receptors (about 90% less than Estradiol), whereas it has a relatively strong binding affinity to vaginal estrogen receptors (equal to Estradiol).
This means that after a single dose of Estriol, the binding to the endometrial estrogen receptor is too short to induce true proliferation, while its binding to the vaginal estrogen receptor is sufficient to exert a strong, heath promoting effect. Estriol is thought to be the most beneficial estrogen for the vagina, vulva and cervix. In cases of postmenopausal vaginal dryness and atrophy, which predisposes a woman to vaginal and bladder inflammation and infection, Estriol supplementation is the most effective and safest estrogen to use. Don’t be fooled by “sexy” TV advertising, drugs like Vagifem and Osphena are barely any better than placebo, yet carry serious, life-threatening side-effects. You owe it to yourself to find a doctor who will test your hormones and prescribe the safe and effective natural female hormone that can take away the pain of menopause and restore the pleasure of intimacy.

